NIEL’S Medical Device
As a digital AI-based assistant, NEIL’S Medical device supports Medical Device Industry in the creation of Clinical Evaluation of the Regulatory document and allows efficient review by competent authorities.
An MDR compliant solution
Fit notified bodies requirements
With the transition from MDD to MDR, the medical device industry needs to demonstrate more clinical evidence which is a challenge for all CE marked devices on the market.
|
|
Manufacturers will need to generate and provide more in-depth clinical data to prove safety and performance claims including tighter equivalency standards. |
|
|
The new regulation requires to most companies to update their clinical data. |
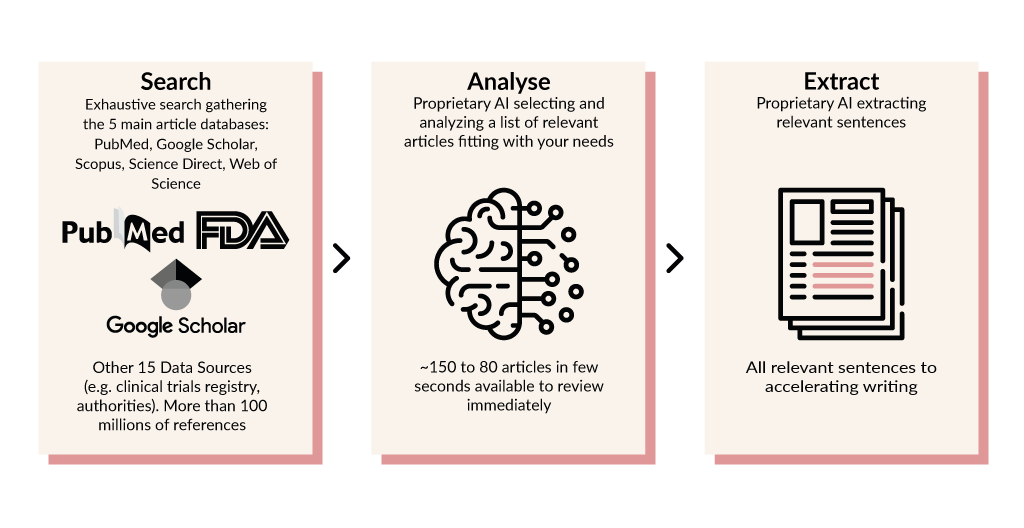
Niel’s Medical device generates this additional clinical data with trusted evidence and automatic updates. A complete data cleaning in order to avoid any repetition which makes the extraction ready to analyse. Save time, effort, and make your clinical documentation approved by competent authorities. |

|
Niel’s Medical device generates this additional clinical data with trusted evidence and automatic updates. A complete data cleaning in order to avoid any repetition which makes the extraction ready to analyse. Save time, effort, and make your clinical documentation approved by competent authorities.

A user friendly platform
Refocus your time on expertise
Niel’s Medical Device tool gains time on added-value research and allows to concentrate on reading and writing your regulatory CER documents